When selecting irrigation, does your choice provide:
Irrisept Antimicrobial Wound Lavage
Self-contained jet lavage with low concentration CHG as a preservative in the bottled solution.
Don’t let your best efforts get diminished by the worst microorganisms...
…because they’re everywhere: the environment, skin, and other endogenous flora.1 These invisible microbes can impact your patients’ care. Even though they’re out of sight, they’re never out of mind, making your choice of wound irrigation a critical one.
Do Your Best Against the Worst—
Add Irrisept to your irrigation plan.
- Low pressure irrigation to remove foreign materials, cellular debris and bacterial contaminants2
- A proven preservative, like CHG, that offers broad spectrum activity against a variety of microorganisms in the bottled solution3
- An extensive safety profile including in-vivo and in-vitro studies and RCT data4-5
- Evidence, including independent and 3rd party research3-13
- Ease of use; a simple device that’s ready to use in seconds
Do Your Best Against the Worst—
Add Irrisept to your irrigation plan.
When selecting irrigation, does your choice provide:
- Pressurized irrigation to remove foreign materials, cellular debris and bacterial contaminants2
- A proven preservative, like CHG, that offers broad spectrum activity against a variety of microorganisms in the bottled solution3
- An extensive safety profile including in-vivo and in-vitro studies and RCT data.4
- Evidence, including independent and 3rd party lresearch3-13
- Ease of use; a simple device that’s ready to use in seconds
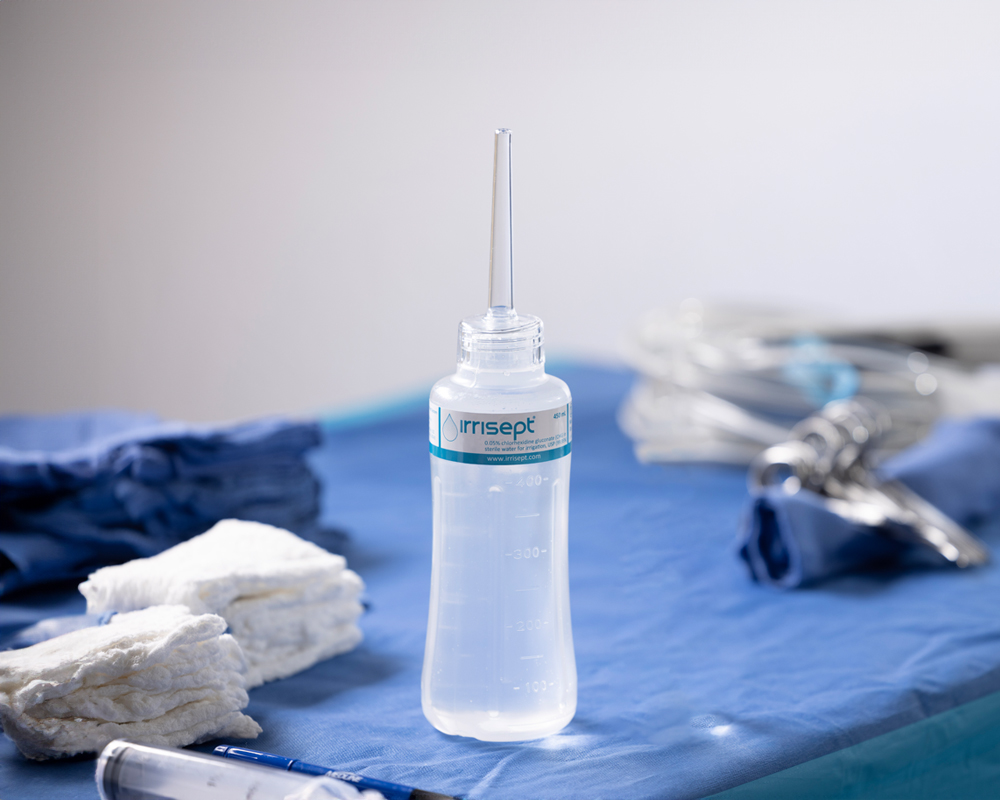
What if a simple irrigation device could impact your patient’s care?
Irrigation can mechanically remove debris and bacterial contaminants. As part of proper wound management, the reduction of bioburden, gives wounds a better chance to heal.2
Irrisept is a self-contained jet lavage delivering low-pressure irrigation. It contains Chlorhexidine Gluconate (CHG), a preservative, to offer broad spectrum activity against various microorganisms in the bottled solution.3
Irrisept is intended for mechanical cleansing and removal of debris, dirt, and foreign materials, including microorganisms from wounds.
Do not use this product if the patient is allergic to chlorhexidine gluconate. Discontinue use immediately if irritation, sensitization, or allergic reaction occurs.
For full product details, refer to product labeling.
Where can Irrisept make a difference?
Irrisept Antimicrobial Wound Lavage can be used across the continuum of care in a variety of healthcare settings.
Pre-Hospital & Field Care
Use Irrisept as a frontline defense to cleanse and remove debris from wounds before hospital treatment, in pre-hospital healthcare settings.
Acute Wound Care
For all types of wounds. Trusted by over 4,000 U.S. hospitals for their irrigation needs.14
Post-Acute Wound Care
Use Irrisept for wound care and cleansing in post-acute settings, such as outpatient clinics, skilled nursing facilities, and other settings where care by a physician is given.
Why Irrisept?
- 0.05% CHG, the right concentration
Irrisept provides low concentration CHG in 99.95% Sterile Water for Irrigation, USP. At this concentration, CHG is non-cytotoxic while demonstrating effectiveness as a preservative against a variety of gram-positive and gram-negative bacteria, fungi, and some viruses in the bottled solution.3-4 - Irrigation with pressure
Irrisept can be manually compressed to deliver low pressure irrigation per the American College of Surgeon’s recommendations.15 It is intended for mechanical cleansing and removal of debris, dirt and foreign materials including microorganisms from wounds. - Backed by testing and independent research
Irrisept has undergone extensive testing, including 3rd party research, and is referenced by name and/or concentration in many independent publications.3-13 - A safety profile like no other
Irrisept is non-cytotoxic and features extensive safety testing, including in-vivo and in-vitro studies and RCT data.3-5 - Designed with ease of use in mind
Irrisept comes in multiple sizes and packaging configurations. Shipped ready to open and use—no mixing, dispensing, or diluting necessary.
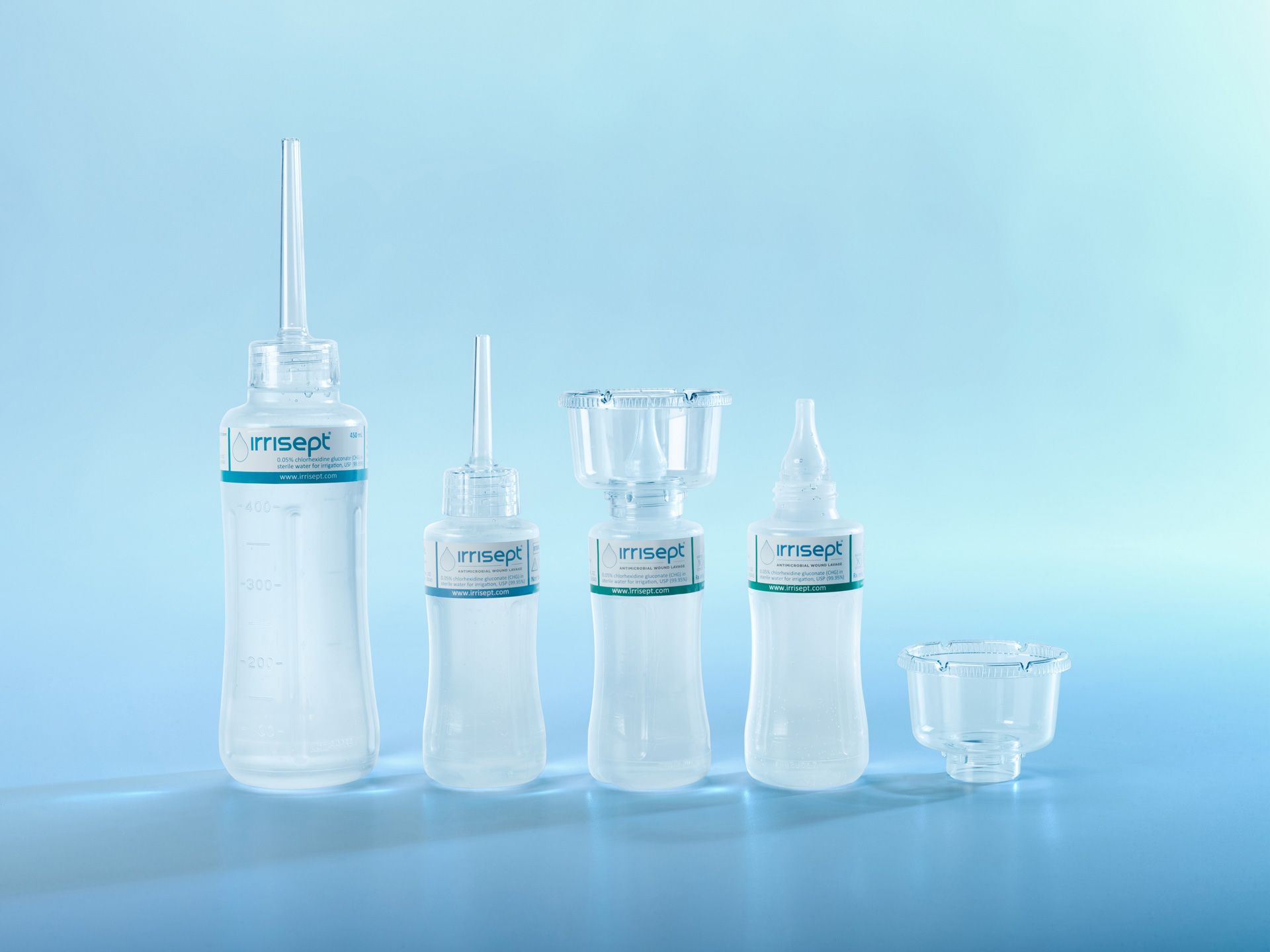
A New Standard of Care
Ready to upgrade your wound management plan?
Irrisept is trusted by more than 4,000 U.S. hospitals for their irrigation needs.14 Contact a rep today to learn more about how Irrisept can make a difference in your patient care.
About Irrisept
Irrisept products are manufactured by Irrimax Corporation, a global medical device company that focuses on reducing infections and healthcare costs while improving patient outcomes. We are committed to supporting research and providing clinical evidence and education to our customers. Our team is made up of experienced members of the medical device industry and Irrimax is consistently ranked among the highest in its peer group in product quality, service levels, and customer support.

References
- 1. Bowler, P. et al. (2001). Wound Microbiology and Associated Approaches to Wound Management. Clin Micro Review, 244- 269.https://doi10.1128/CMR.14.2.244-269.2001
- 2. Lewis, K., & Pay, J. (2021, January). Wound Irrigation. NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK538522/
- 3. (2022). KTK Summary. Doc. 537161 V5 Evaluation of CHG as a preservative in the solution
- 4. Biocompatibility Matrix. Data on file at Irrimax Corp. Lawrenceville, GA
- 5. (2022). Protocol CLP-01: An Independent Review of Safety Data From a Closed Clinical Study Using Irrisept (Protocol # IRR-CT-901-2013-01)
- 6. Spencer et al. (2017). Reduction in Colon Surgical Site Infections Using CHG Irrigant Solution [Conference Presentation]. AORN 2017, Boston, MA
- 7. Truitt, K., & Kleinheinz, S. (2017). Target Zero: Eliminating Surgical Site Infection With 0.05% CHG Jet Lavage Irrigation [Conference Presentation]. AORN 2017, Boston, MA
- 8. Dotson, N., Rasheid, S., Marcet, J., & Sanchez, J. (2015). In Irrigation of Incisions With 0.05% CHG Reduces Surgical Site Infections in Colorectal Surgery [Conference Presentation]. ASCRS 2015, Boston, MA
- 9. Merceron et al. (2019). Comparison of Complications Following Implant-Based Breast Reconstruction Using Triple Antibiotic Solution Versus Low Concentration Chlorhexidine Gluconate Solution. Mod Plas Surg, 09(04), 74–85. https:// doi.org/10.4236/mps.2019.94010
- 10. Mangold et al. (2019). Standardising Intraoperative Irrigation with 0.05% Chlorhexidine Gluconate in Caesarean Delivery to Reduce Surgical Site Infections: A Single Institution Experience. J Peri Practice, 30(1-2), 24–33. https://doi.org/10.1177/1750458919850727
- 11. Lung et al. (2022). Chlorhexidine Gluconate Lavage During Total Joint Arthroplasty May Improve Wound Healing Compared to Dilute Betadine. J Exp Ortho, 9(1). https://doi.org/10.1186/s40634-022-00503-w
- 12. Driesman et al. (2020). Perioperative Chlorhexidine Gluconate Wash During Joint Arthroplasty has Equivalent Periprosthetic Joint Infection Rates in Comparison to Betadine Wash. J Arthroplasty, 35(3), 845–848. https://doi.org/10.1016/j.arth.2019.10.009
- 13. Frisch et al. (2017). Intraoperative Chlorhexidine Irrigation to Prevent Infection in Total Hip and Knee Arthroplasty. Arth Today, 3(4), 294–297. https://doi.org/10.1016/j.artd.2017.03.005
- 14. Data on file at Irrimax Corp., Lawrenceville, GA
- 15. Ashley et al. (2014). Acute Wound Care. ACS Surgery: Princ Prac . (7th ed., pp. 215–216). Decker Intellectual Properties Inc.

